Proton Exchange Membrane Fuel Cells (PEMFCs) are a type of fuel cell that use hydrogen as a fuel source to generate electrical power. In this article, we will discuss how PEMFCs work in combination with hydrogen to produce clean and efficient energy.
Overview of PEMFCs
PEMFCs are a type of electrochemical device that convert the chemical energy of hydrogen and oxygen into electrical energy. They consist of several key components, including an anode, a cathode, and a proton exchange membrane (PEM).
The anode is the negative electrode, where hydrogen is oxidized to produce protons and electrons. The cathode is the positive electrode, where oxygen is reduced to form water. The PEM separates the anode and cathode compartments and allows only protons to pass through, creating a flow of electric current.
PEMFCs are known for their high efficiency, low operating temperature, and rapid start-up times. They are also lightweight and have a low profile, making them suitable for a wide range of applications, including transportation, portable electronics, and stationary power generation.
How PEMFCs Work with Hydrogen
PEMFCs operate by combining hydrogen and oxygen to produce electrical power. Here is a step-by-step breakdown of how PEMFCs work with hydrogen:
- Fuel Supply
Hydrogen fuel is supplied to the anode compartment of the fuel cell. The hydrogen can come from a variety of sources, including natural gas, methanol, or electrolysis of water.
- Anode Reaction
At the anode, the hydrogen molecules are split into protons and electrons in a process known as oxidation. The electrons are released and flow through an external circuit, producing electrical power. The protons are conducted through the PEM to the cathode compartment.
- Cathode Reaction
At the cathode, oxygen from the air is supplied and reduced to form water. This reaction consumes the protons and electrons from the anode reaction, completing the circuit.
- Electrical Power
The flow of electrons through the external circuit generates electrical power, which can be used to power a variety of devices and systems.
- Water Production
The only byproduct of the PEMFC reaction is water, which is produced at the cathode. The water can be collected and reused or released as waste.
Advantages of Using PEMFCs with Hydrogen
PEMFCs have several advantages when used with hydrogen as a fuel source. These include:
- Clean Energy
PEMFCs produce only water as a byproduct, making them a clean and environmentally friendly energy source. They do not produce greenhouse gases or harmful pollutants, making them an important technology for combating climate change and air pollution.
- High Efficiency
PEMFCs have high efficiency, meaning they convert a high percentage of the chemical energy of hydrogen into electrical power. This makes them a cost-effective and energy-efficient technology for a variety of applications.
- Low Operating Temperature
PEMFCs operate at relatively low temperatures, around 80°C, which allows for faster start-up times and greater efficiency. This also means that they do not require as much heat management as other types of fuel cells, making them a more practical and cost-effective technology for many applications.
- Versatility
PEMFCs are a versatile technology that can be used in a variety of applications, including transportation, portable electronics, and stationary power generation. Their lightweight and low profile make them suitable for a wide range of devices and systems.
Challenges and Future of PEMFCs with Hydrogen
Despite their many advantages, there are several challenges to the widespread adoption of PEMFCs with hydrogen. One challenge is the need for a reliable and efficient hydrogen storage and distribution infrastructure. Currently, hydrogen is mainly produced from
fossil fuels, such as natural gas, which limits the potential for hydrogen fuel cells to truly be a clean and sustainable energy source.
Another challenge is the cost of producing and maintaining PEMFCs. While the cost has decreased over time, it is still relatively high compared to other energy technologies. This limits the practicality and accessibility of PEMFCs for many applications.
Despite these challenges, the future of PEMFCs with hydrogen looks promising. Researchers and engineers are continually working to improve the efficiency and reliability of PEMFCs, as well as develop new technologies for hydrogen production and storage.
In addition, PEMFCs are already being used in a variety of applications, including powering electric vehicles and providing backup power for buildings. As the demand for clean energy continues to grow, PEMFCs with hydrogen are poised to become an increasingly important technology in the transition to a more sustainable future.
 The Toyota Mirai is a hydrogen fuel cell vehicle that was first introduced to the market in 2014. It is a groundbreaking car that has the potential to revolutionize the way we think about transportation, particularly in terms of reducing greenhouse gas emissions. In this article, we will explore the technology behind the Toyota Mirai and how it works to provide a sustainable and efficient mode of transportation.
The Toyota Mirai is a hydrogen fuel cell vehicle that was first introduced to the market in 2014. It is a groundbreaking car that has the potential to revolutionize the way we think about transportation, particularly in terms of reducing greenhouse gas emissions. In this article, we will explore the technology behind the Toyota Mirai and how it works to provide a sustainable and efficient mode of transportation. It has been more than 50 years now since General Motors unveiled the first hydrogen car. An engine running entirely on clean energy? Even more, outputting drinkable water? A deal almost too good to be true. However, it is also too good to give up on easily. Still, in the last few years, electric vehicles (EVs) have taken the “green” market almost on its entire. So, who killed the hydrogen car?
It has been more than 50 years now since General Motors unveiled the first hydrogen car. An engine running entirely on clean energy? Even more, outputting drinkable water? A deal almost too good to be true. However, it is also too good to give up on easily. Still, in the last few years, electric vehicles (EVs) have taken the “green” market almost on its entire. So, who killed the hydrogen car? The lighter-than-air craft
The lighter-than-air craft  Actually, there’s more stored energy or explosive power in a car’s tank of gasoline than there is a tank of hydrogen. More importantly, gasoline splashes, and pools, and volatises (evaporates) whereas Hydrogen goes straight up.
Actually, there’s more stored energy or explosive power in a car’s tank of gasoline than there is a tank of hydrogen. More importantly, gasoline splashes, and pools, and volatises (evaporates) whereas Hydrogen goes straight up. Nowadays of course we all use the standard notation pH, so that we make consistent references and avoid confusion. So, what are we discussing when the pH symbol is bandied about?
Nowadays of course we all use the standard notation pH, so that we make consistent references and avoid confusion. So, what are we discussing when the pH symbol is bandied about?
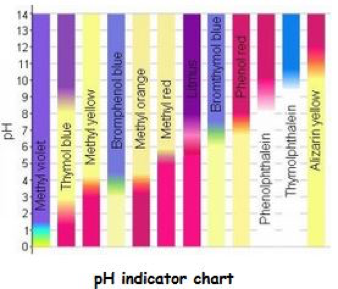 This chart gives an idea of chromatic responsiveness of several reagents. Of course there are others that are not included here. The centre strip that is half red and half purple shows the original litmus responsiveness in comparison to the greater accuracy of 11 other types.
This chart gives an idea of chromatic responsiveness of several reagents. Of course there are others that are not included here. The centre strip that is half red and half purple shows the original litmus responsiveness in comparison to the greater accuracy of 11 other types.